Director: Kathleen Sheehan, PhD
Staff: Rebecca Andrews & Chelsea Mannie
Phone (consult & pricing): 314-362-8744
Phone (scheduling & data analysis): 314-7362-7448
Fax: 314-747-1227
Email: mAbcore@wustl.edu
Location: West Building, Room 4724
Overview
The Washington University School of Medicine Hybridoma Center (WUHC) provides monoclonal antibody technology to the University research community. With over 35 years of experience, the Hybridoma Center assists investigators in the generation of custom monoclonal antibody-producing B cell hybridomas.
Initially, guidance is provided by the Director in the custom selection of appropriate species to be used, development of immunization schedules, screening protocols, and methods for antibody production. Immunized spleen cells derived from either mice or Armenian hamsters are fused and maintained at the Hybridoma Center.
Once sufficient growth has occurred, culture supernatants are screened by ELISA and/or provided to the investigator for screening. Hybridomas secreting antigen-specific antibodies are cloned to establish a stable line secreting a unique antibody with defined specificity and isotype. After a second round of cloning, the antibody producing cultures are expanded and returned to the investigator for further processing. Frozen vials are maintained in liquid nitrogen by the Hybridoma Center.
The WUHC also provides custom monoclonal antibody development for parties outside of the university. Contact the director at sheehan@wustl.edu for further details.
Access
Service available to: All entities, including for-profit organizations.
Priority service given to: Washington University.
Additional Information: Contracted services are open to non-University organizations on a fee for service basis.
Request services
The Washington University School of Medicine Hybridoma Center is under the faculty supervision of Kathleen Sheehan, PhD and is operated by Research Technicians Rebecca Andrews and Chelsea Mannie. Dr. Sheehan may be contacted at 314-362-8744 for information regarding immunization procedures, screening strategies, general operation of the Center or special needs.
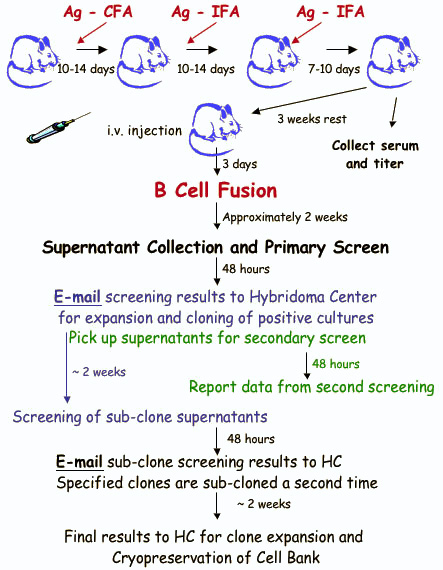
Rebecca and Chelsea should be contacted at 314-362-7448 to schedule fusions and communicate screening results. We recommend that fusions be scheduled at the time animals are initially immunized, as this will minimize any delays for the investigators. Fusions are performed on Tuesdays and Thursdays. All animals to be fused must demonstrate a positive serum titer of > 1:1000. Titer results are reviewed by the Director prior to fusion.
Services
The mouse or Armenian hamster host to be fused may be obtained, immunized, boosted and bled by the investigator or can be contracted through the Hybridoma Center. For B cell fusions the murine myeloma P3X63Ag8.653 is used.
On the scheduled fusion day, the investigator will bring the host animal to the Hybridoma Center – West Building, Room 4724. The animals should be brought to the Center by 9:30 a.m., unless alternate plans have been previously arranged. The animals should be clearly marked (preferably ear tagged) and transferred to the Hybridoma Center holding cage. If the investigator is late, the fusion may be cancelled at the discretion of the Hybridoma Center.
After the fusion is performed, the Hybridoma Center will notify the investigator when to expect supernatants for screening. This will generally be 10 to 14 days after the fusion. The investigator will receive replicate 96 well plates containing approximately 125 ul per well of culture supernatant. Researchers should be prepared to screen 300-1000 individual supernatants from the fusion of a single mouse spleen. ELISA screening may also be contracted to the Hybridoma Center.
Investigators will be given 2 days to notify the Center which supernatants are positive (this limitation is due to the exponential growth of cells in small culture volumes). A second set of supernatants will be provided to verify antigen-reactive wells. Positive cultures will be expanded and 4 to 7 days later another set of supernatants will be given to the investigator. Only cell lines which have tested positive 2 times will be frozen and subcloned. (Note: Hamster hybridomas are notoriously unstable and will be cloned immediately from original culture wells). In a typical fusion, a maximum of 12 lines will be frozen, with the best 6 to be subcloned. Results from all screenings are expected within 2 days. If this poses a problem for the investigator, the Hybridoma Center can try to accommodate researchers if contacted in advance. Additional lines may be subcloned and frozen for an additional fee
Select positive clones will be subcloned by limiting dilution to ensure clonality and/or select for higher antibody production. These cultures will be tested in a manner similar to the original supernatants. Three subclones from each line will be frozen.
The Hybridoma Center can generate and provide investigators with additional volumes of supernatants. Source may be from an active cell line or thawed line. Volumes vary depending on investigators’ needs.
All cell lines generated remain the property of the individual investigator. Researchers may choose to store frozen vials at the Hybridoma Center or all vials can be transferred to the original investigator upon completion of the fusion. The Hybridoma Center services are most successful when there is full communication between the Center and the investigator. For our own records, the Hybridoma Center requests that we be informed as to the success of each particular fusion (in the eyes of the researcher), the utility of individual hybridomas, and informed of any publications documenting hybridomas produced in the facility.
Monoclonal antibody isotype analysis of up to 10 samples simultaneously can be performed.
If desired, the HC will ship frozen cell lines, supernatants and other related items on behalf of the investigator.
Protocols
Antigens
- Proteins – 10 – 100 μg per injection, per animal
- Cells – 0.5 – 5.0 x 107 cells per injection, per animal
- DNA – 100 μg per injection, per animal
Adjuvant
- Freund’s Adjuvant
- Alum
Immunization schedule
| Day | Manipulation | Adjuvant | Site |
| 0 | Primary Immunization | CFA | s.c. |
| 14 | Boost #1 | IFA | s.c. |
| 28 | Boost #2 | IFA | s.c. |
| 36 | Serum Collection | ||
| 42 | Rest Prior to Fusion (or Boost #3) | >IFA | s.c. |
| 56 | i.v. Boost | none | i.v. |
| 59 | Harvest Spleen and Fuse |
Materials
P3X63Ag8.653 murine myeloma (maintained at < 1 x 106/ml)
50% w/v PEG 1500, warmed to 37° C
Medium
IMDM (4g/L glucose) supplemented with 20% fetal bovine serum, 4 mM L-glutamine, 1 mM sodium pyruvate, 50 U penicillin, 50 μg streptomycin and 50 μM 2-ME in the absence or presence of HAT or HT for selection, and Hybridoma Cloning Factor (1% final).
Cell preparation
- Tease spleen in ice-cold serum-free medium (I-0). Pass cell suspension through a Falcon 70 micron cell filter and suspend in 50 ml of ice-cold I-0. Centrifuge and wash cells three times at 4° C in I-0. Resuspend cells after the third wash in 10 ml I-0 and count viable cells. Keep cells on ice.
- Concurrently with the spleenocytes, centrifuge and wash myeloma cells three times, using I-0 and resuspend in I-0. Count viable cells.
- Add an appropriate number of myeloma cells to the entire volume of spleen cells according to the following ratios and centrifuge together.
| Species | Spleenocytes: Myeloma |
| mouse – mouse | 5:1 |
| hamster – mouse | 4:1 |
Fusion protocol
Aspirate all supernatant and suspend pellet by tapping the end of the tube. Place the tube in a container of warm water (37°C). Gradually, over a period of 60 seconds, add 1 ml of 37° C 50% w/v PEG while tapping the side of the tube to achieve thorough mixing. Over the next 60 seconds continue to mix. Dilute the PEG/cell mixture slowly by adding 2 ml of I-0 drop-wise over a 2-minute time span. Next, use a 10 ml pipet and add 8 ml 37° C IMDM containing 10% FBS over a 4 minute period. Bring the total volume to 50 ml using I-10. Centrifuge at 4°C and resuspend in medium at the appropriate volume to bring the cells to the following concentrations:
| Type | Cell Concentration |
| mouse | 1.0 x 106 cells per ml |
| hamster | 0.6 x 106 cells per ml |
Add 150 μl added per well. An additional 50 μl of a 4X HAT solution can be added at 16-24 hours after fusion.
Feeding schedule
The day of the fusion is considered day 0. Fusion plates are examined at 24-48 hours for any abnormalities (i.e. bacterial contamination). On day 7, wells are inspected visually and then fed. One half of the volume in each well is aspirated using a sterile pasteur pipet. A new pipet is used for each plate. Wells are fed with 125 μl of I-20 + 2ME + HAT on days 7, 11 and thereafter as needed, i.e. Mon., Wed., Fri.
Cultures are examined visually at each feeding. Once a majority of wells appear 50% confluent for growth, supernatants are harvested for screening. Plates are fed at this time.
**Investigators should provide information on the serum titer of the immunized animal. If the titer is greater than 1:10,000, it may be important to feed the cultures at least 3-4 times prior to the screening. This will essentially “wash away” any antibody release from dying B cells and decrease background Ig levels.
Protocol references
Sheehan KCF, Lai KS, Dunn GP, Bruce AT, Diamond MS, Hentel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ and Shreiber RD. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR1) from mice immunized by vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006. 26:804-19.
Sheehan KCF. (2007) Production of Monoclonal Antibodies. In Making and using antibodies: a practical handbook. Eds GC Howard and MR Kaser. (73-94). Boca Raton, Fl. CRC Press.
Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine Tumor Necrosis Factors. J Immunol 142:3884-3893, 1989.
Sheehan KC, Calderon J, Schreiber RD. Generation and characterization of monoclonal antibodies specific for the human IFN gamma receptor. J Immunol 140:4231-4237, 1988.
Sanchez-Madrid F, Szklut P, Springer TA. Stable hamster-mouse hybridomas producing IgG and IgM hamster monoclonal antibodies of defined specificity. J Immunol 130:309-312, 1983.
Kohler G, Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497, 1975
Approximately 10 – 14 days following the fusion, at a time when the majority of culture wells are nearly 50% confluent, supernatants are collected from each well for screening. The investigator will be notified one or two days prior to supernatant harvest. The investigator should be prepared to screen 300 -1000 individual wells. About 125 μl of supernatant is harvested from each well. Screening results are required within 48 hours to insure that positive cultures do not overgrow or die.
Prior to fusion and screening the investigator should fine tune their screening strategy, using control serum and immune serum. This is the time to be sure that all reagents are available and that proper dilutions of secondary reagents are known. The method of screening should be designed to identify hybridomas secreting antibodies that are specific and will function in the manner desired by the researcher. Common screening protocols include: ELISA, Western Blot Analysis, Immunoprecipitation of radiolabeled protein, Ligand Binding Inhibition, FACS or Bioassay screen for the desired function. Dr. Sheehan is available to discuss these methods; a few factors to consider are as follows:
- Are suitable quantities of antigen/cells available to test individual wells, or will samples need to be pooled and then retested?
- Is the screening assay specific? If an animal is immunized with a fusion protein, can the investigator differentiate between reactivity to the antigen vs. carrier? Has the investigator monitored reactivity to both transfected and non-transfected cells?
- Can the screening method be completed and analyzed within two days?
- Is a two-tiered screening method needed? One to identify antigen specific hybridomas and another to isolate mAb with specific functions or subclasses.
- Are the appropriate secondary reagents identified and titered? This is particularly important when screening Armenian hamster fusions. Choose secondary reagents that bind only IgG species to avoid identification of IgM secreting mAbs.
- Will non-Ig components in the culture supernatant interfere with the read out of the assay? For example, if a proliferation assay is used, the high concentration of thymidine in the selection medium will prevent incorporation of 3H-thymidine in dividing cells. Often the fetal calf serum present in supernatants at 20% (v/v) will pose a problem.
- Remember – you only get what you screen for. Antibodies that bind recombinant protein may not necessarily block function or work for immunohistochemisty. Multi-tiered screening assays are essential to identify the best mAb.
General information
Important characteristics of monoclonal antibodies
- Specificity
- Isotype
- Affinity for antigen
- Stability
Applications of monoclonal antibodies
- Enumeration or identification of cell populations
- Cell depletion
- Cell isolation
- Protein purification
- Immunoassays for quantitation of soluble or cell surface antigens
- Probing the structure or function of soluble or cell surface antigens
- Diagnosis in cancer or infectious disease
- Imaging
Advantages
- Homogeneity: Monoclonal antibody represents a single antibody molecule that binds to antigens with the same affinity and promote the same effector functions.
- Specificity: The product of a single hybridoma reacts with the same epitope on antigens.
- Immunizing Antigen: Need not be pure or characterized and is ultimately not needed to produce large quantities of antibody.
- Selection: It is possible to select for specific epitope specificities and generate antibodies against a wider range of antigenic determinants.
- Antibody Production: Unlimited quantities of a single well-defined monospecific reagent.
Disadvantages
- Affinity: Average affinity of monoclonal antibodies are generally lower than polyclonal antibodies.
- Effector Functions: Because antibody is monoclonal, it may not produce the desired biologic response.
- Specificity: Monoclonals against conformational epitopes on native proteins may lose reactivity with antigens that have been minimally perturbed.
- Crossreactions: Antibodies sometimes display unexpected crossreactions with unrelated antigens.
- Time and effort commitment: Very large.
- Armenian hamsters are phylogenetically distinct from mice and respond well to murine, as well as other species, proteins and yet are able to fuse productively with murine myeloma lines. This model system has been used extensively to develop high affinity neutralizing reagents to murine cytokines, receptors and transcription factors.
- Armenian hamster mAbs generally exhibit nM binding to antigen.
- Armenian hamster mAbs can be easily purified by Protein A affinity chromatography.
- Armenian hamster mAbs are non-immunogenic when injected into mice and are ideal for use in in vivo models of disease.
- Armenian hamster mAb can exhibit a circulatory half life of 4 – 14 days in wild type mice.
Three decades of Hybridoma Center publications
With nearly thirty years in operation, The Washington University School of Medicine Hybridoma Center has played a central role in scientific discovery. Our expertise in the development of both murine and Armenian hamster monoclonal antibodies has been documented in significant publications, across multiple disciplines and biologic systems.
Selected publications
Hourcade DE, Akk A, Mitchell LM, Zhou H-F, Haubart R, Pham CTN. Anti-complement activity of the Ixodes scapularis salivary protein Salp20. Mol. Immunol. 2016; 69:62-9. PMCID: PMC4698246
Yan H, Zhou H-F, Akk A, Hu Y, Springer LE, Ennis TL, Pham CTN. Neutrophil proteases promote abdominal aortic aneurysm via NET formation and type I interferon induction. Arterioscler. Thromb. Vasc. Biol. 2016; 36:1660-9. PMCID: PMC4965335
Morrissey JJ, Mellnick VM, Luo J, Siegel MJ, Figenshau RS, Bhayani S, Kharasch ED. Evaluation of Urine Aquaporin 1 and perilipin 2 concentrations as biomarkers to screen for renal cell carcinoma. JAMA Oncology 1: 204-212, 2015, doi:10.1001/jamaoncol.2015.0213. PMID: 26181025.
Bertram P, Akk A, Zhou H-F, Pham CTN, Hourcade DE. Anti-mouse properdin TSR 5/6 monoclonoal antibodies block complement alternative pathway-dependent pathogenesis. Monoclon. Antib. Immunodiagn. Immunother. 2015; 34:1-6. PMCID: PMC4350452
Siebrasse EA, Pastrana DV, Nguyen NL, Wang A, Roth MJ, Holland SM, Freeman AF, McDyer J, Buck CB, Wang D. WU polyomavirus in respiratory epithelial cells from lung transplant patient with Job syndrome. Emerg Infect Dis. 2015 Jan;21(1):103-6. doi: 10.3201/eid2101.140855. PMID: 25531075 PMCID: PMC4285236
Siebrasse EA, Nguyen NL, Smith C, Simmonds P, Wang D. Immunohistochemical detection of KI polyomavirus in lung and spleen. Virology. 2014 Nov;468-470:178-84. doi: 10.1016/j.virol.2014.08.005. Epub 2014 Sep 3. PMCID: PMC4254350
Zhou H-F, Yan H, Bertram P, Hu Y, Springer LE, Thompson RW, Curci JA, Hourcade DE, Pham CTN. Fibrinogen-specific antibody induces abdominal aortic aneurysm in mice through complement lectin pathway activation. Proc. Natl. Acad. Sci. USA 2013, 110: E4335-4344. PMCID: PMC3831981
Zhou H-F, Yan H, Cannon JL, Springer LE, Green JM and Pham CTN. CD43-mediated IFN gamma production by CD8+ T cells promotes abdominal aortic aneurysm in mice. J. Immunol. 2013, 190:5078-5085. PMCID: PMC3647012
Bishnupuri, KS, Sainathan, SK, Bishnupuri, K, Leahy, DR, Luo, Q, Anant, S, Houchen CW, Dieckgraefe, BK. Reg4-Induced Mitogenesis Involves Akt-GSK3–Catenin Signaling in Human Colorectal Cancer. Molecular Carcinogenesis. Epup 2013 October 2; 53(Suppl. 1):169-180. PMID: 24151146
Ndonwi M, Girard TJ, Broze GJ Jr. The carboxy-terminus of TFPI alpha is required for its interaction with factor V and Va. J. Thromb. Haemost. 2012; 10:1944-1946. PMC3462013.
Ladenson, JH. Reflections on the evolution of cardiac biiomarkers. 2012 Clinical Chem 58:21-24.
Wice BM, Reeds DN, Tran HD, Crimmins DL, Patterson BW, Dunai J, Wallendorf MJ, Ladenson JH, Villareal DT, Polonsky KS. Xenin-25 amplifies GIP-mediated insulin secretion in humans with normal and impaired glucose tolerance but not type 2 diabetes. Diabetes. 2012 Jul;61(7):1793-800. Epub 2012 Apr 20.
Macy EM, Crimmins DL, McQuillan JJ, Griest TA, Brada NA, Tarawneh R, Lee JM, Ladenson JH. Reversal of EDTA Interference in the Measurement of Visinin-Like Protein 1. Clin Chem. 2012 Apr 5.
Tarawneh R, D’Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, Fagan AM, Head D, Mintun MA, Ladenson JH, Lee JM, Morris JC, Holtzman DM. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011 Aug;70(2):274-85. PMCID: PMC3154071
Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, Antignac C, Pollak M, Kopp JB, Winn MP, Shaw AS. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest. 2011 Oct;121(10):4127-37. PMCID: PMC3195463
Bishnupuri KS, Luo Q, Sainathan SK, Kikuchi K,Sureban SM, Sabarinathan M, Gross JH, Aden K, May R, Houchen CW, Anant S, Dieckgraefe BK. Reg IV regulates normal intestinal and colorectal cancer cell susceptibility to radiation-induced apoptosis. Gastroenterology. 2010 Feb;138(2):616-26, 626.e1-2.
Hadwiger G, Dour S, Arur S, Fox P, Nonet ML. 2010. A Monoclonal Antibody Toolkit for C. elegans. PLoS ONE 5(4): e10161. PMCID: PMC2854156
Wice BM, Wang S, Crimmins DL, Diggs-Andrews KA, Althage MC, Ford EL, Tran H, Ohlendorf M, Griest TA, Wang Q, Fisher SJ, Ladenson JH, Polonsky KS. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem. 2010 Jun 25;285(26):19842-53. Epub 2010 Apr 26.
Liszewski MK, Leung MK, Hauhart R, Fang CJ, Bertram P, Atkinson JP. Smallpox inhibitor of complement enzymes (SPICE): dissecting functional sites and abrogating activity. J Immunol. 2009 Sep 1;183(5):3150-9. Epub 2009 Aug 10. PMID: 19667083
Cai SF, Fehniger TA, Cao X, Mayer JC, Brune JD, French AR, Ley TJ. Differential expression of granzyme B and C in murine cytotoxic lymphocytes. J Immunol. 2009 May 15;182(10):6287-97. PMCID: PMC2714542
Gracheva E.O., Hadwiger G., Nonet M.L., amd J.E. Richmond. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynpatic density. Neurosci. Lett., 444:137-142. 2008. PMCID: PMC2585374
Lee JM, Blennow K, Andreasen N, Laterza O, Modur V, Olander J, Gao F, Ohlendorf M, Ladenson JH. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008 Oct;54(10):1617-23. Epub 2008 Aug 14.
Huang S, Gilfillan S, Kim S, Thompson B, Wang X, Sant AJ, Fremont DH, Lantz O, Hansen TH.MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J Exp Med. 2008 Apr 28. PMID: 18443227
Uppaluri R, Sheehan KCF, Wang L, Bui JD, Brotman JJ, Lu B, Gerard C, Hancock WW and Schreiber RD. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation 2008; 86:137-47. PMID 18622291 NIHMS83976
Katpally U, Wobus CE, Dryden K, Virgin HW 4th, Smith TJ. Structure of antibody-neutralized murine norovirus and unexpected differences from viruslike particles. J Virol. 2008 Mar;82(5):2079-88. Epub 2007 Dec 19. PMID: 18094184
Lenschow, D.J., C. Lai, N. Frias-Staheli, N.V. Giannakopoulos, A. Lutz, T. Wolff, A. Osiak, B. Levine, R.E. Schmidt, A. Garcia-Sastre, D.A. Leib, A. Pekosz, K.P. Knobeloch, I. Horak, and H.W. Virgin. 2007. ISG15 functions as a critical antiviral molecule against both influenza and herpes viruses. Proceedings of the National Academy of Sciences, USA. 104:1371-1376. PMID: 17227866
Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, Ladenson JH. Identification of novel brain biomarkers. Clin Chem. 2006. Sep;52(9):1713-21. Epub 2006 Jul 20.
Sheehan KCF, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006. 26:804-819. PMID: 17115899
Schwulst, S.J., Grayson, M.H. DiPasco, P.J., Davis, C.G., Brahmbhatt, T.S., Ferguson, T.A., & Hotchkiss, R.S. 2006. Agonistic monoclonal antibody against CD40 receptor decreases lymphocyte apoptosis and improves survival in sepsis. Journal of Immunology, 177(1), 557-65.
Bui, J.D., Carayannopoulos, L.N., Lanier, L.L., Yokoyama, W.M., & Schreiber, R.D. 2006. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol, 176, 905-13.
Lenschow, D.J., N. Giannakopoulos, L.J. Gunn, C. Johnston, A.K. O’Guin, R.E. Schmidt, B. Levine, and H.W. Virgin. 2005. Identification of ISG15 as an antiviral molecule during Sindbis virus infection in vivo. Journal of Virology. 79:13974-13983. PMID: 16254333
Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004 Dec;2(12):e432. PMID: 15562321
Divine, JK, Flake, N, Sheehan K and Gottlieb DI. Expression of a novel antigen, EEM-1, in the mouse embryo and embryonic stem cell-derived embryoid bodies. Developmental Dynamics 2000;218:207-211.
Weaver CH, Sheehan KCF and Keath EJ. Localization of a yeast phase specific gene product to the cell wall in Histoplasma capsulatum. Infect and Immun 1996;64:3048-3054.
Meraz MA, White JM, Sheehan KCF, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell JD, Carver-Moore K, DuBois RN, Clark R, Aguet M and Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 1996;84:431-442.
Sheehan KCF, Pinckard JK, Arthur CD, Dehner LP, Goeddel DV and Schreiber RD. Monoclonal antibodies specific for murine p55 and p75 TNF receptors: Identification of a novel in vivo role for p75. J. Exp. Med. 1995;181:607-617.
Bodor GS, Porter S, Landt Y, Ladenson JH. Development of monoclonal antibodies for an assay of cardiac troponin I and preliminary results in suspected cases of myocardial infarction. Clin Chem 1992;38:2203-14.
Rogers HW, Sheehan KCF, Brunt LM, Dower SK, Unanue ER and Schreiber RD: Interleukin-1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci USA 1992;89:1011-1015.
Silva DP, Jr, Landt Y, Porter SE, Ladenson JH. Development and application of monoclonal antibodies to human cardiac myoglobin in a rapid fluorescence immunoassay. Clin Chem 1991;37:1356-1364.
Hogquist KA, Nett MA, Sheehan KCF, Pendleton KD, Schreiber RD and Chaplin DD: Monoclonal antibodies to murine interleukin-1. J Immunol 1991;146:1534-1540.
Sheehan KCF, Ruddle NH and Schreiber RD: Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol 1989;142:3884-3893.